How to utilize CRISPR library to speed up your research?
With the rapid development of CRISPR/Cas9 gene editing technology, CRISPR library screening has played an important role in drug screening, viral infection, and tumor functional gene screening experiments. Compared with cDNA libraries and RNAi libraries, CRISPR library screening has the advantages of versatility, low noise, high knockout efficiency, and low off-target rate, making it a popular method for candidate gene screening.
Q: What is CRISPR library screening?
A: CRISPR library screening is a method for accurate screening for functional genes.
Q: What’s the difference between two plasmid library and one plasmid library?
One plasmid system: It includes promoter, gRNA, Cas9, and selection marker. gRNA and Cas9 can be simultaneously transferred into cells. However, due to the large size of the one plasmid system, its infection efficiency is low.
Two plasmid system: only carries gRNA and the promoter; Cas9 stable cell line generation is needed. The two plasmid system has higher efficiency and is more convenient to modify the plasmid, but requires two transductions.
In this article, we will introduce the procedure and tips of two-plasmid system CRISPR library screening. Let’s get started now!
Procedure
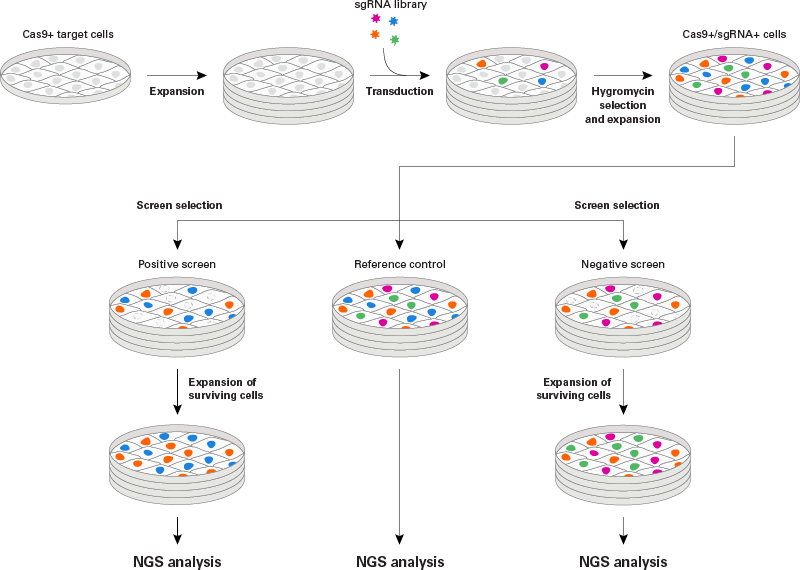
1.Generation of Cas9 stable cell line
1) Cas9 lentivirus packaging;
2) Lentivirus transduction and Cas9 expressing stable cell clones isolation and screening;
3) Cas9 activity test.
2. sgRNA library construction (pre-made or customized)
Design 3-6 sgRNAs for each gene, then each sgRNA clones to a plasmid backbone, and then amplify the plasmid library and NGS sequencing.
3. sgRNA library lentivirus packaging
1) Mix sgRNA library and helper plasmids and transfect 293T cells in T225 culture flask;
2) After 48 hours, collect culture media and store in -80℃.
4. Lentivirus transduction (take A549 as an example)
1) Test for A549 kill curve, and determine the optimal concentration of puromycin for screening;
2) Transduce 2×10^7 A549-Cas9 cells with 0.3 MOI (i.e. 6×10^6 cells are transduced), each sgRNA in this library integrates to >1000 cells.
5. Cell phenotype screening (take A549 as an example)
Divide the whole genome knockout cell pool into two parts.
1) One is the experimental group to exert screening pressure, such as virus infection, drug treatment, etc;
2) The other one is the control group. Screening cells based on phenotypes such as drug resistance, proliferation ability, and survival ability.
Note: There are positive screening and negative screening according to different screening purposes. Generally 3 control groups and 3 experimental groups are needed to lower the statistic error.
6.NGS sequencing and data analysis
Perform the following steps on the experimental group and the control group:
1) Genome extraction;
2) PCR amplification of sgRNAs;
3) NGS sequencing;
4) Bioinformatics analysis (MaGCKFlute).
7. Gene function study
Validate gene function from two aspects: the necessity and sufficiency.
1) Knockout each candidate gene;
2) Overexpression or rescue experiment.
Tips
The keys to successful CRISPR library screening:
1) Selecting a high cutting efficiency Cas9 stable cells is the key to obtain a KO cell library with high coverage rate.
2) Screening range: for high-throughout screening, the size of a library is one of the important aspects. Thus, try to choose small libraries and prioritize pre-made libraries.
3) During lentivirus transduction, ensure one lentivirus particle enter one cell.
Above is the whole procedure for CRISPR library screening. Would you like to give it a shoot and get your own CRISPR library project? Contact us to get a quote :)
EDITGENE
EDITGENE provides one-stop CRISPR library screening service. We provide over 200 types of Cas9 stable cell lines, pre-made human/mouse libraries, customized library service.
Let us do the job, and provide you the trustworthy data!

