[Literature Review] CRISPR Screening Reveals Aging Regulators in Neural Stem Cells
The adult mammalian brain contains several regions with neural stem cells (NSCs) that can generate new neurons and repair damage caused by strokes or injuries. Among these, the subventricular zone (SVZ) is the most active NSC niche, where quiescent NSCs are stored.
These cells can be activated to divide, producing progenitor cells that migrate to the olfactory bulb and differentiate into neurons. However, as aging progresses, NSC activity significantly declines, leading to sensory and cognitive impairments. Identifying genes that influence NSC activation could help develop interventions to mitigate age-related brain function decline.
Currently, genetic interventions focus on only a few genes, and CRISPR-Cas9 technology has not yet been applied to normal aging cells. Developing such a screening platform could potentially uncover gene regulatory mechanisms that enhance tissue function, offering new insights for addressing the decline in regenerative capacity and cognitive function in the aging brain.

Original Article Link: https://doi.org/10.1038/s41586-024-07972-2
To systematically identify genes that promote NSC activation with aging, researchers developed a genome-wide CRISPR-Cas9 knockout screening platform in primary NSC cultures from both young and old mice. Their findings, published in Nature under the title "CRISPR-Cas9 Screens Reveal Regulators of Ageing in Neural Stem Cells", aimed to uncover genes that influence aging in neurogenic niches.
The platform is efficient and scalable, enabling researchers to directly study key regulators of cellular aging in vivo. It is suitable for different cell types in aged mice and is compatible with other CRISPR-Cas9 techniques.By targeting aging tissues, these genetic interventions could reveal novel strategies to delay or reverse aging, providing new insights for addressing cognitive and regenerative decline related to aging and neurodegenerative diseases.
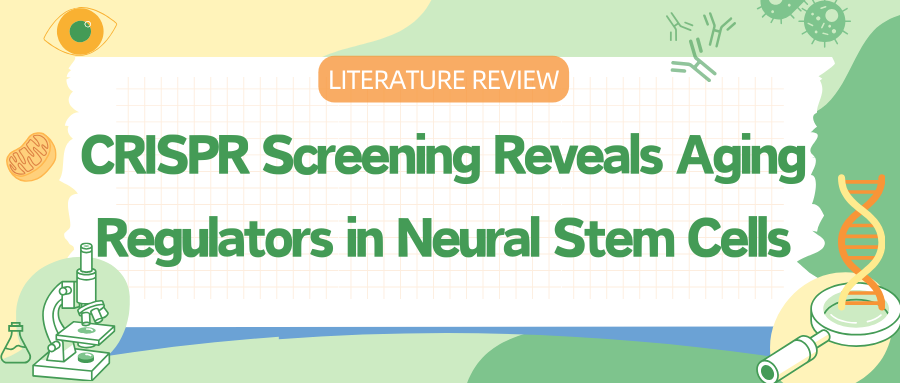
I. CRISPR-Cas9 Screen to Boost Old NSCs
Primary NSCs can switch between quiescent (qNSC) and activated (aNSC) states in response to different growth factors. However, NSCs from older mice have reduced activation capacity, reflecting aging processes in the body. To establish a screening platform, researchers used Cas9-expressing mice and isolated NSCs from the subventricular zone for genome-wide CRISPR-Cas9 knockout screening. More than 400 million quiescent NSCs were transduced with a library targeting around 23,000 protein-coding genes.
After activation, the cells underwent high-throughput sequencing to identify genes that promote or inhibit activation. The results revealed 654 genes associated with enhanced activation and 1,386 genes linked to inhibited activation. The study also found significant differences in activation responses between young and old NSCs.
Gene ontology analysis showed that genes enhancing the activation of old NSCs were related to cilium organization and glucose transport, highlighting the new role of glucose metabolism in aging NSCs. This research provides a comprehensive dataset that uncovers key gene knockouts promoting the transition from a quiescent to activated state, offering new insights into the aging mechanisms of neural stem cells.
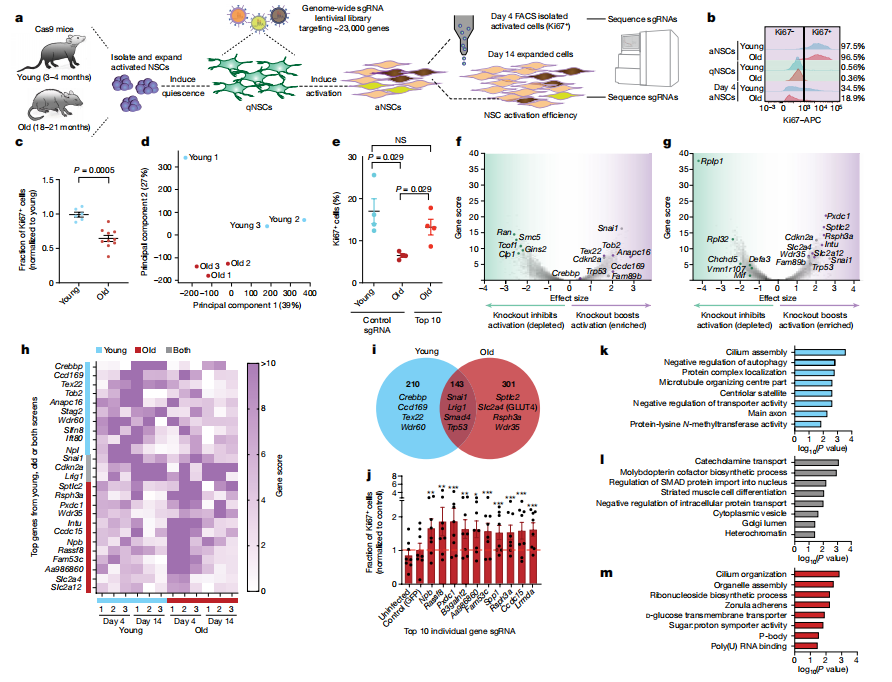
Fig 1. A genome-wide screen identifies 300 gene knockouts that boost old NSC activation
II. CRISPR-Cas9 Screening in Old Brains
Researchers used a gene knockout screening platform to target the subventricular zone (SVZ) in old Cas9 mice. Lentiviruses expressing sgRNAs and mCherry were injected into the lateral ventricles to target NSCs. Five weeks later, gene knockouts were confirmed, with NSC progeny migrating to the olfactory bulb. This method leveraged the regenerative properties of the SVZ to assess how specific gene knockouts affect NSC activation and neurogenesis.
Using various sgRNA libraries, 24 of 50 gene knockouts showed significant enrichment in the olfactory bulb, enhancing old NSC function. Some knockouts predicted to hinder activation showed slight depletion, reflecting reduced neurogenesis in aged mice.
This in vivo screening platform identified key genes involved in NSC aging, including those related to cilia, glucose metabolism, and ribonucleoproteins. It provides insights into genetic factors affecting NSC function and opens avenues for potential therapeutic interventions.
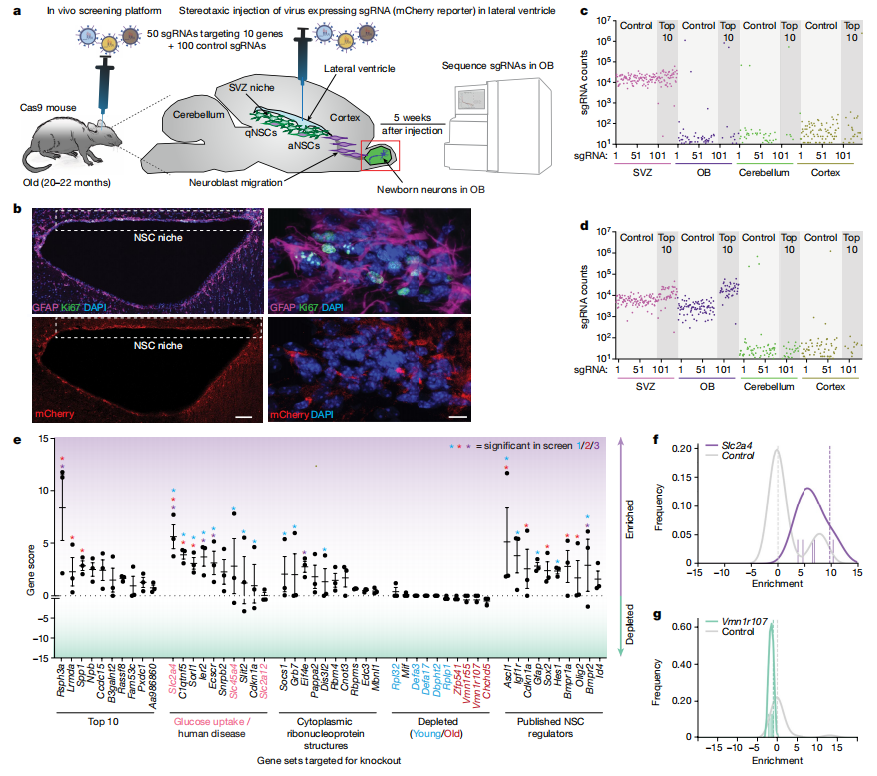
Fig 2. In vivo screening platform for rapid testing of gene knockout effects on NSC activation
III. Knocking Out GLUT4 Boosts Neurogenesis
The knockout of glucose transporter GLUT4 (Slc2a4) has emerged as an effective strategy to enhance the function of old NSCs both in vitro and in vivo. This study aimed to determine whether reducing GLUT4 expression in the subventricular zone (SVZ) could promote neurogenesis in aged individuals.
Researchers used stereotaxic injection of sgRNAs targeting Slc2a4 into the ventricles of old Cas9 mice, successfully reducing GLUT4 levels in the SVZ and olfactory bulb five weeks post-injection. Weekly EdU injections were used to track newly generated cells. The results showed a significant increase in mCherry+ newborn cells in the olfactory bulb, with many expressing neuronal markers like NeuN and TUJ1, confirming that Slc2a4 knockout enhances neurogenesis.
Immunofluorescence analysis revealed increases in both quiescent NSCs (qNSCs) and activated NSCs (aNSCs) in the SVZ niche, suggesting that GLUT4 depletion promotes symmetric cell division without depleting the NSC population. Additionally, GLUT4 expression was found to increase with age in both NSCs and astrocytes, indicating a potential detrimental effect of GLUT4 on NSC function as aging progresses. These findings suggest that the age-related rise in GLUT4 may hinder neurogenesis, making GLUT4 knockout a promising strategy to enhance NSC activity and neurogenesis in older individuals.
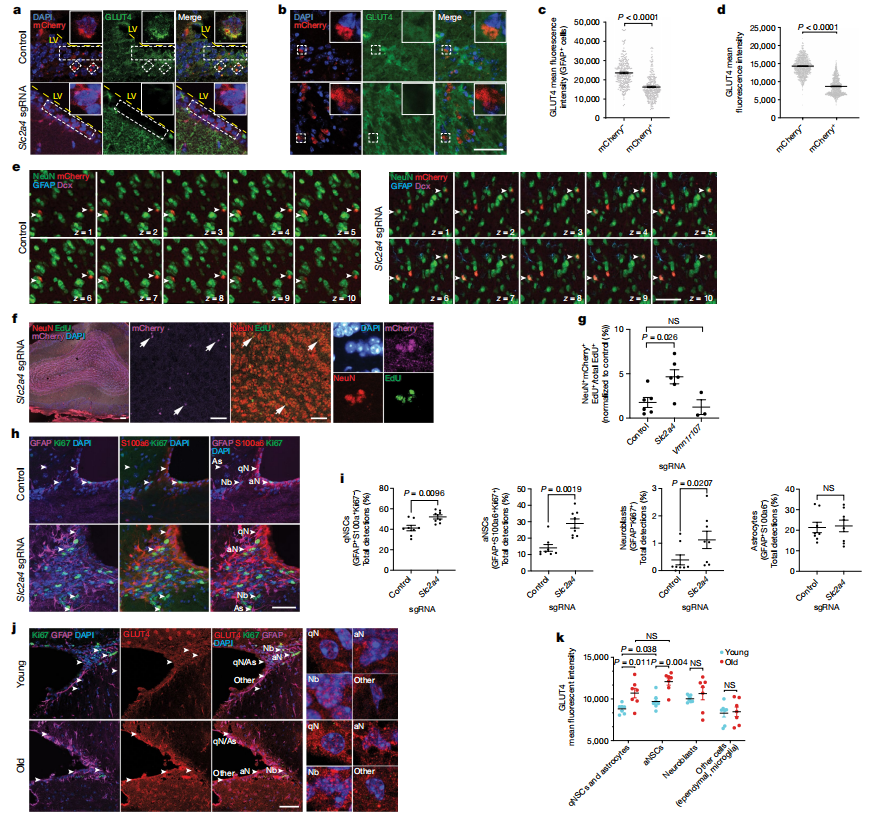
Fig 3. Slc2a4 knockout in the SVZ NSC niche boosts neurogenesis in old mice
IV. Old NSCs Exhibit High Glucose Uptake
Aged neural stem cells (NSCs) show increased glucose uptake primarily through the transporter GLUT4, which is upregulated with age. Researchers analyzed genome-wide in vitro screening data to investigate components of the glucose uptake pathway in the context of NSC aging. The results showed that knockout of the Slc2a4 gene significantly enhanced the activation of old NSCs in vitro and boosted neurogenesis in vivo. In contrast, Slc2a12 knockout did not yield similar effects.Single-cell RNA sequencing revealed that only Slc2a4 was significantly upregulated in quiescent NSCs (qNSCs) with aging. Immunofluorescence staining confirmed a modest increase in GLUT4 levels in aged qNSCs. Additionally, knockout of Stx4a, a protein involved in GLUT4 transport, also promoted the activation of old NSCs.
Despite increased glucose uptake, aged qNSCs showed heightened glycolytic activity and reduced mitochondrial respiration, suggesting a metabolic shift favoring glucose as an energy source. Limiting glucose availability revealed that Slc2a4 knockout and short-term glucose starvation significantly promoted the activation of old qNSCs. Treatment with 2-deoxy-d-glucose, a glycolysis inhibitor, further stimulated activation in aged qNSCs.
These findings suggest that high GLUT4 levels and glucose uptake may contribute to the quiescent state of aged NSCs. Overall, the study highlights the potential for targeting glucose metabolism through genetic or dietary interventions to improve NSC function in older populations, emphasizing the importance of metabolic pathways in regulating stem cell activity during aging.
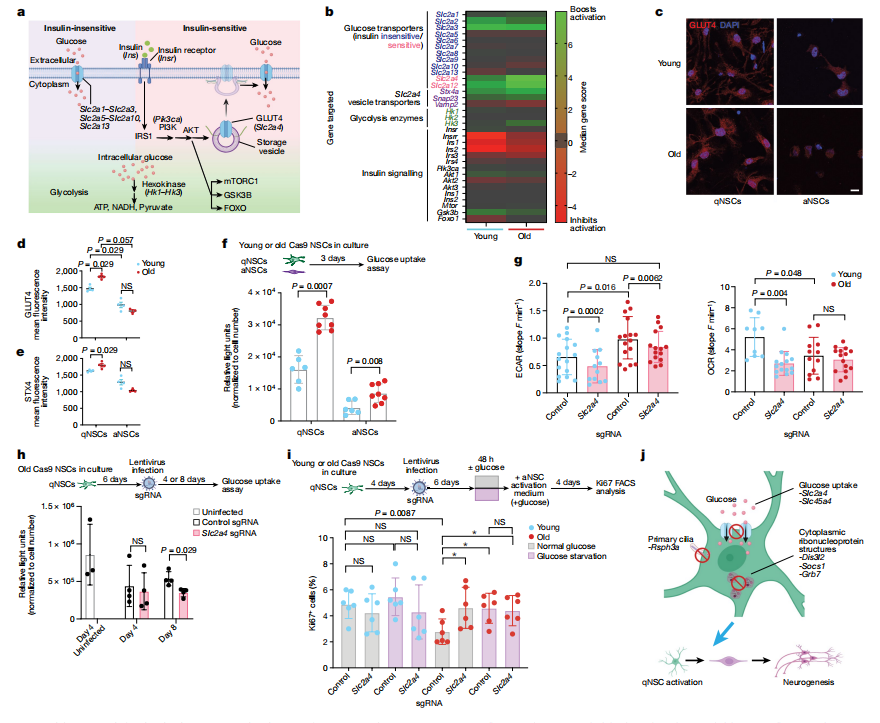
Fig 4. Old NSCs exhibit high glucose uptake that can be targeted to improve activation
In conclusion, by using genome-wide CRISPR-Cas9 knockout screening, researchers identified over 300 genes that enhance the activation of old NSCs in vitro when disrupted. The findings indicate that increased glucose uptake, potentially linked to dysregulated insulin-glucose signaling, may be a common feature of cellular aging, with implications for neuronal dysfunction and Alzheimer's disease.
Targeting this glucose uptake through GLUT4 knockout or glucose restriction offers promising strategies to counter aging. The establishment of an in vivo screening platform provides a scalable method to discover new genetic interventions that could rejuvenate aged tissues, particularly in the brain, and help address cognitive decline and neurodegenerative diseases. Overall, this approach underscores the potential of genetic and environmental interventions to delay or reverse aspects of aging.
EDITGENE provides a one-stop complete solution for CRISPR library screening , including CRISPR KO/CRISPRa/CRISPRi , along with the most comprehensive collection of plasmids/viruses available. Ready-to-ship within one week—place your order and start screening!
Recent Blogs:
Follow us on social media
Contact us
+ 833-226-3234 (USA Toll-free)
+1-224-345-1927 (USA)
info@editxor.com






