[Literature Review] CRISPR Knockout: A Breakthrough in Nrf2 Activation for Kidney Disease Treatment

Acute kidney injury (AKI) is a significant global healthcare problem associated with increased mortality and morbidity. Ischemia-reperfusion injury (IRI), resulting from a temporary reduction of kidney blood flow, is a major cause of AKI. However, currently, there is no specific therapy available for AKI. Immune cells, particularly CD4+ T cells, play a crucial role in the pathogenesis of early injury and repair processes in kidney IRI.
The nuclear factor erythroid 2-related factor 2/kelch-like ECH-associated protein 1 (Nrf2/Keap1) pathway is a promising therapeutic target for kidney diseases. Nrf2 is a transcription factor that controls multiple antioxidant genes, while Keap1 regulates its cytoplasmic levels. Under oxidative stress, the association between Keap1 and Nrf2 is disrupted, leading to increased nuclear translocation of Nrf2 and upregulation of numerous antioxidant genes.
The role of Nrf2 in kidney diseases is under-investigated, pharmacological Nrf2 activators have been explored in clinical trials but faced challenges with adverse events. Previous studies have demonstrated the protective effects of Nrf2 activation in kidney IRI; however, the role of T cell-specific Keap1 deletion still remains to be fully elucidated.
Original Article Link: https://doi.org/10.1089/ars.2022.0058
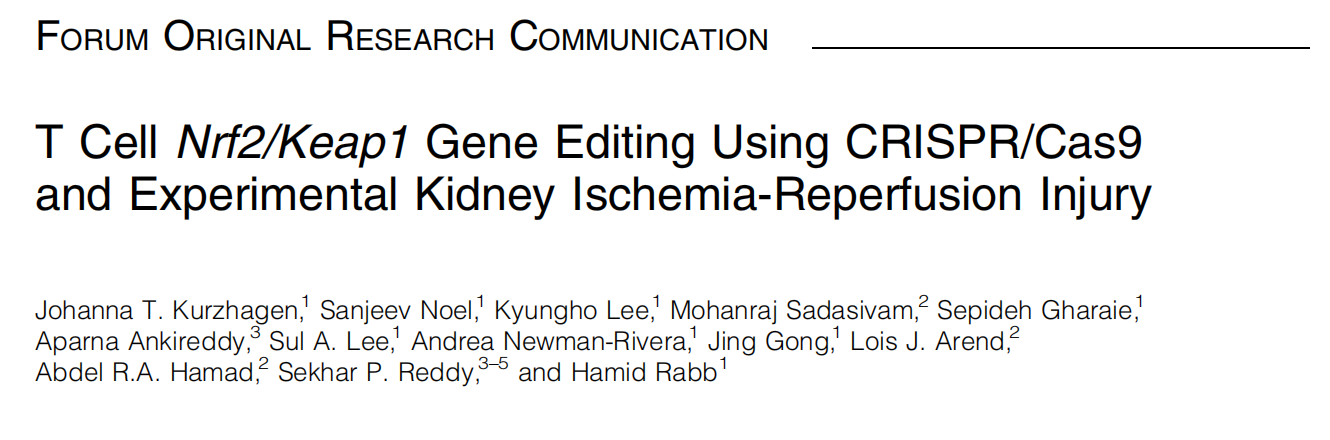
Given the crucial role of T cells in kidney IRI, T cell-specific Keap1 deletion is a promising therapeutic target. To investigate the mechanism by which CRISPR-mediated Nrf2/Keap1 pathway protects the kidney against IRI, researchers from Johns Hopkins University School of Medicine have conducted a series of experiments, demonstrating that CRISPR/Cas9-mediated gene editing enhances Nrf2 activity in CD4+ T cells, making it a novel therapeutic approach for renal IRI. Their findings were published in the journal Antioxid Redox Signal (IF:5.9) under the title "T Cell Nrf2/Keap1 Gene Editing Using CRISPR/Cas9 and Experimental Kidney Ischemia-Reperfusion Injury".
CRISPR/Cas9 gene editing is a powerful targeted genetic modification technology that has been successfully used for Keap1 knockout (KO) in human T cells, leading to the upregulation of Nrf2 target genes. The researchers discovered that T cell-specific Keap1 deletion in mice (CD4-Keap1-knockout [KO]) provides structural and functional protection against kidney IRI. Moreover, adoptive transfer of T cells with increased Nrf2 activity from Keap1-KO mice protected wild-type mice from kidney IRI.
This study demonstrated that ex vivo Keap1-KO using CRISPR/Cas9 in murine CD4+ T cells upregulated Nrf2-dependent antioxidant target genes, and provided functional protection from kidney IRI.
I. Keap1-KO using CRISPR/Cas9 augmented Nrf2 target gene expression
The researchers employed three distinct sgRNAs, each targeting different sites of the Keap1 gene, which were tested individually or in combination, to investigate the effect of Keap1-KO on Nrf2 target gene expression in murine CD4+ T cells. Primary mouse CD4+ T cells were cultured prior to the delivery of the ribonucleoprotein (RNP) complex, consisting of sgRNA and Cas9. Cells were harvested post-electroporation, and the effect of Keap1-KO was evaluated by quantifying Nrf2 target gene expression.
Quantitative real-time polymerase chain reaction (qRT-PCR) analyses revealed a substantial increase in Nqo1, Hmox1, and Gclc 24 hours after electroporation for all tested sgRNAs, both individually and in combination. sgRNA3 was selected for subsequent experiments, based on its superior ability to elevate Nqo1 mRNA expression and significantly upregulate Hmox1 and Gclc mRNA expression.
Furthermore, the researchers have tested mRNA expression of pro- and anti-inflammatory cytokines, to identify potential immunological alterations caused by Keap1-KO 24 hours after RNP electroporation. A significant upregulation of Tnfa was observed in Keap1-KO CD4+ T cells compared with control cells, while there was no significant difference in Ifng, Il17, and Il10 mRNA expression between Keap1-KO and control CD4+ T cells.
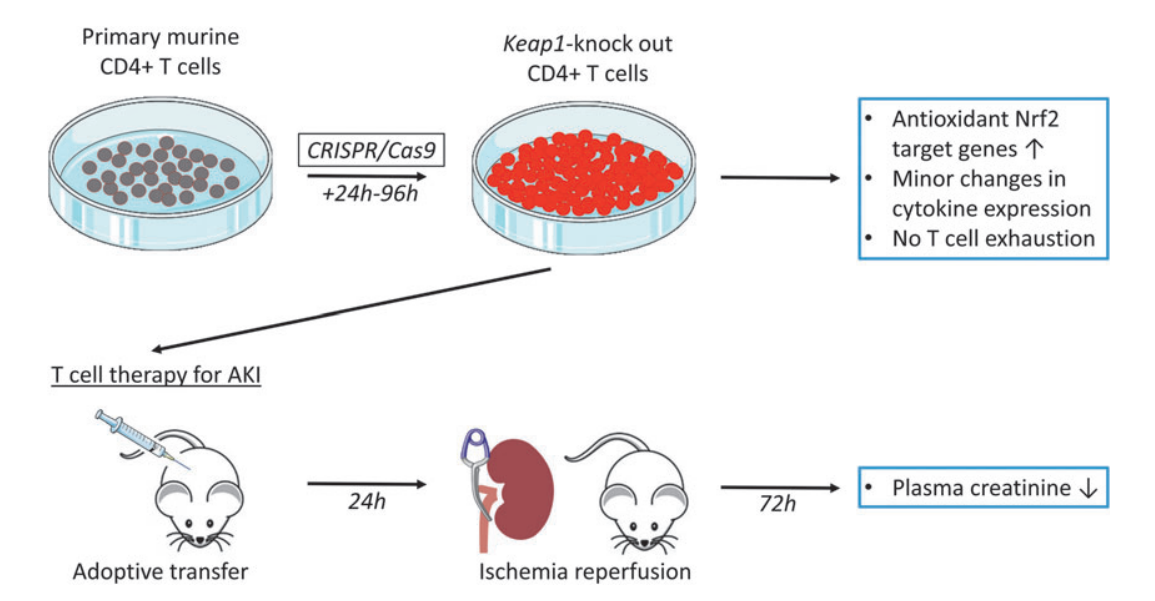
Fig.1 Graphic summary illustration of in vitro Nrf2/Keap1 editing use of CD4+ T cells and their impact on in vivo ischemia-reperfusion-induced AKI in a mouse model
II. Keap1-KO resulted in sustained antioxidant gene expression
To investigate the effect of prolonged culture time on Nrf2 target gene expression in Keap1-KO CD4+ T cells, CD4+ T cells from the control and Keap1-KO groups were cultured up to 96 hours after electroporation. By performing qRT-PCR to assess the antioxidant gene expression, the researchers observed a strong and sustained upregulation of Nrf2 target genes Nqo1, Hmox1, Gclc, and Gclm in Keap1-KO T cells, compared with control cells. The results demonstrated that Keap1-KO using CRISPR/Cas9 led to sustained Nrf2 activation.
III. Immunoblot analysis confirmed Keap1-KO
The researchers analyzed CD4+ T cells by immunoblotting 96 hours after RNP electroporation, to evaluate the effect of CRISPR/Cas9-mediated Keap1 gene editing on Keap1 protein expression. The results revealed significantly reduced Keap1 protein expression in Keap1-edited CD4+ T cells compared with control CD4+ T cells, and, NQO1 protein expression showed 50% higher expression in Keap1-edited cells. These results confirmed that CRISPR/Cas9 successfully abolished KEAP1 protein expression in Keap1-KO CD4+ T cells with subsequent upregulation of Nrf2 target genes in vitro.
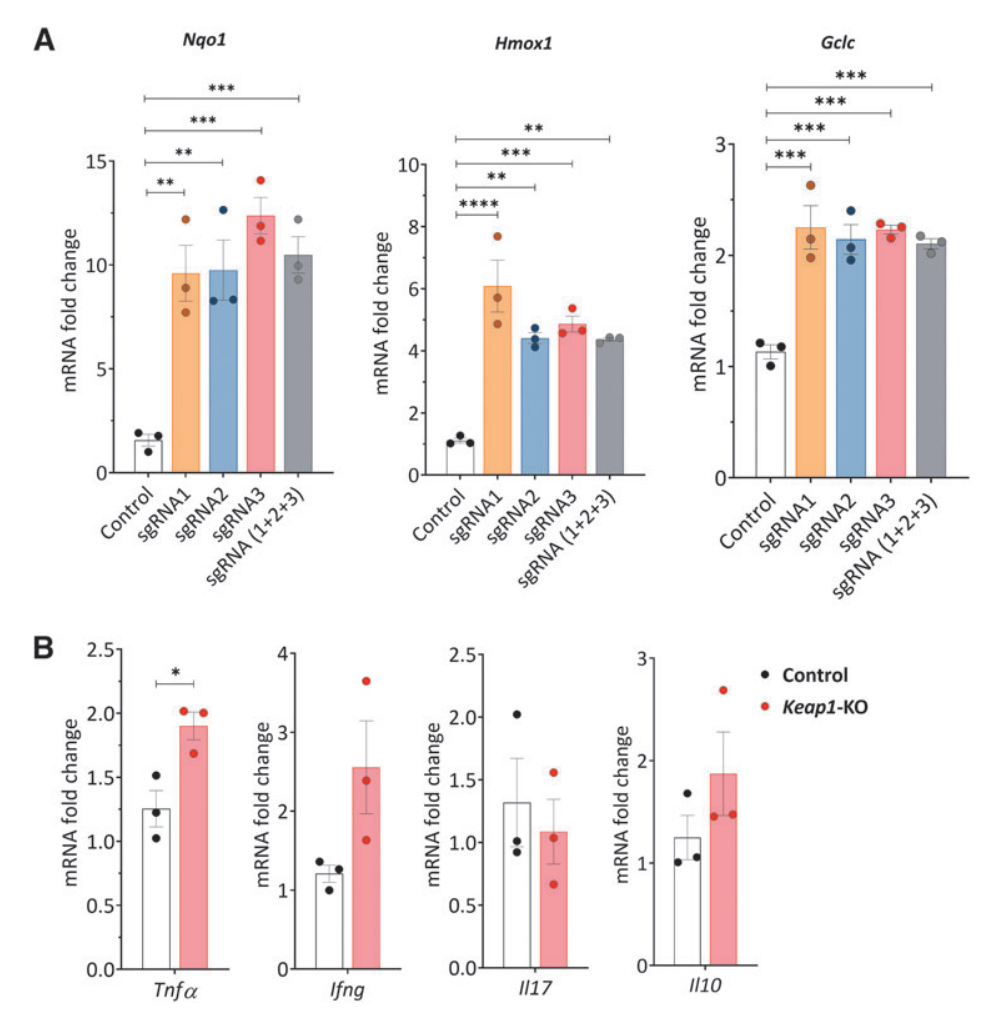
Fig.2 CRISPR/Cas9-mediated Keap1-gene-KO increased Nrf2-regulated antioxidant and cytokine gene expression in cultured murine CD4+ T cells 24 h after electroporation
IV. Sustained Nrf2 augmentation did not induce CD4+ T cell activation or exhaustion
To investigate whether continuous Nrf2 activity induces CD4+ T cell activation or exhaustion, Keap1-KO CD4+ T cells were analyzed using flow cytometry and qRT-PCR. At 72 hour time point, the researchers observed no difference in FoxP3 expression compared with control cells, and activation markers CD25 and CD69 were also comparable between the groups. Additionally, cytokine expression of TNFa, IFNc, IL17, and IL10 between Keap1-KO and control cells no significant difference was observed.
Moreover, to evaluate whether Keap1 knockout by CRISPR/Cas9 results in CD4+ T cell exhaustion in ex vivo culture conditions, the researchers assessed the expression of coinhibitory molecules,. The results showed that no effect was observed on the experimented coinhibitory molecules related to T cell exhaustion in Keap1-KO T cells compared with control groups.
Fig.3 Cytokine and chemokine expression in control and Keap1-KO CD4+ T cells under normoxic and hypoxic in vitro conditions
V. Keap1-KO of CD4+ T cells changed their cytokines and Nrf2 target gene expression in normoxic and hypoxic conditions in vitro
To elucidate the effects of enhanced Nrf2 antioxidant activity on kidney-resident CD4+ T cells during kidney ischemic injury, the researchers exposed the cells to in vitro hypoxic conditions. Compared with control cells, Keap1-KO CD4+ T cells exhibited increased mRNA levels of Nrf2 target genes Nqo1, Hmox1, and Gclm in both normoxia and hypoxia. However, there were no significant changes in hypoxia-inducible factor 1α (Hif1a) and B-cell lymphoma 2 (Bcl2) expression between the groups in normoxic and hypoxic conditions.
Additionally, the researchers performed cytometric bead array (CBA) analysis of culture supernatants from Keap1-KO CD4+ T cells, and revealed significantly reduced IL2 and IL6 levels in Keap1-KO CD4+ T cells, compared with non-edited control cells in both normoxia and hypoxia. Additionally, IFNc levels were significantly increased in hypoxic condition, while Il6 showed a decreased in normoxic condition.
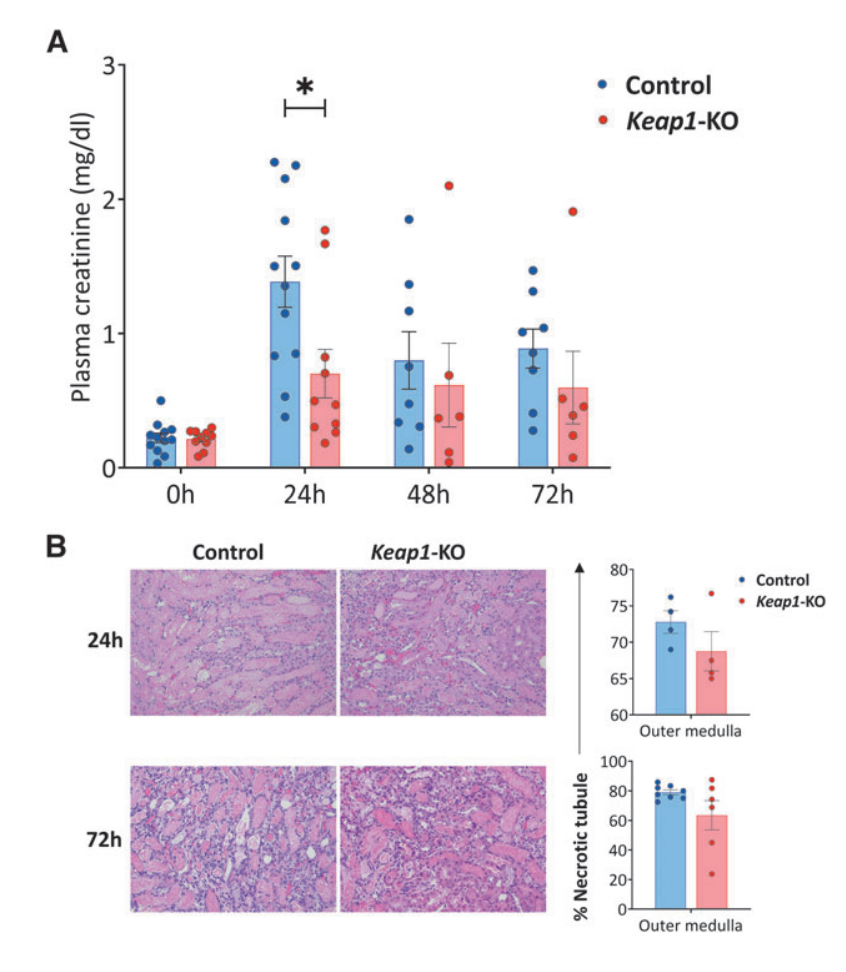
Fig.4 Adoptive transfer of Keap1-KO CD4+ T cells resulted in functional protection from IRI
VI. Keap1-KO CD4+ T cell immunotherapy improved kidney function after IRI
To evaluate the therapeutic potential of Keap1-KO CD4+ T cell immunotherapy in a mouse model of kidney IRI, the researchers adoptively transferred Keap1-KO or control CD4+ T cells into nu/nu mice, followed by IR surgery. Compared with mice that received control CD4+ T cells, the results showed that Keap1-KO CD4+ T cell recipient mice exhibited significantly reduced plasma creatinine levels, while no significant differences in necrotic tubules were found in the outer medulla between the groups.
Moreover, immunophenotyping of recipient kidneys confirmed the successful trafficking of adoptively transferred CD4+ T cells to the kidneys, and there was significantly reduced CD25 expression in Keap1-KO CD4+ T cells compared with control cells, and there was also a trend for lower TNFa levels in Keap1-KO CD4+ T cells.
However, there was no differences in CD69 expression or intracellular levels of IFNc between Keap1-KO cells isolated from post-ischemic kidneys compared with controls. Analysis of CD4+ T cells from kidneys of recipient mice also showed an extremely weak signal for FoxP3, which was likely due to the small number of cells.
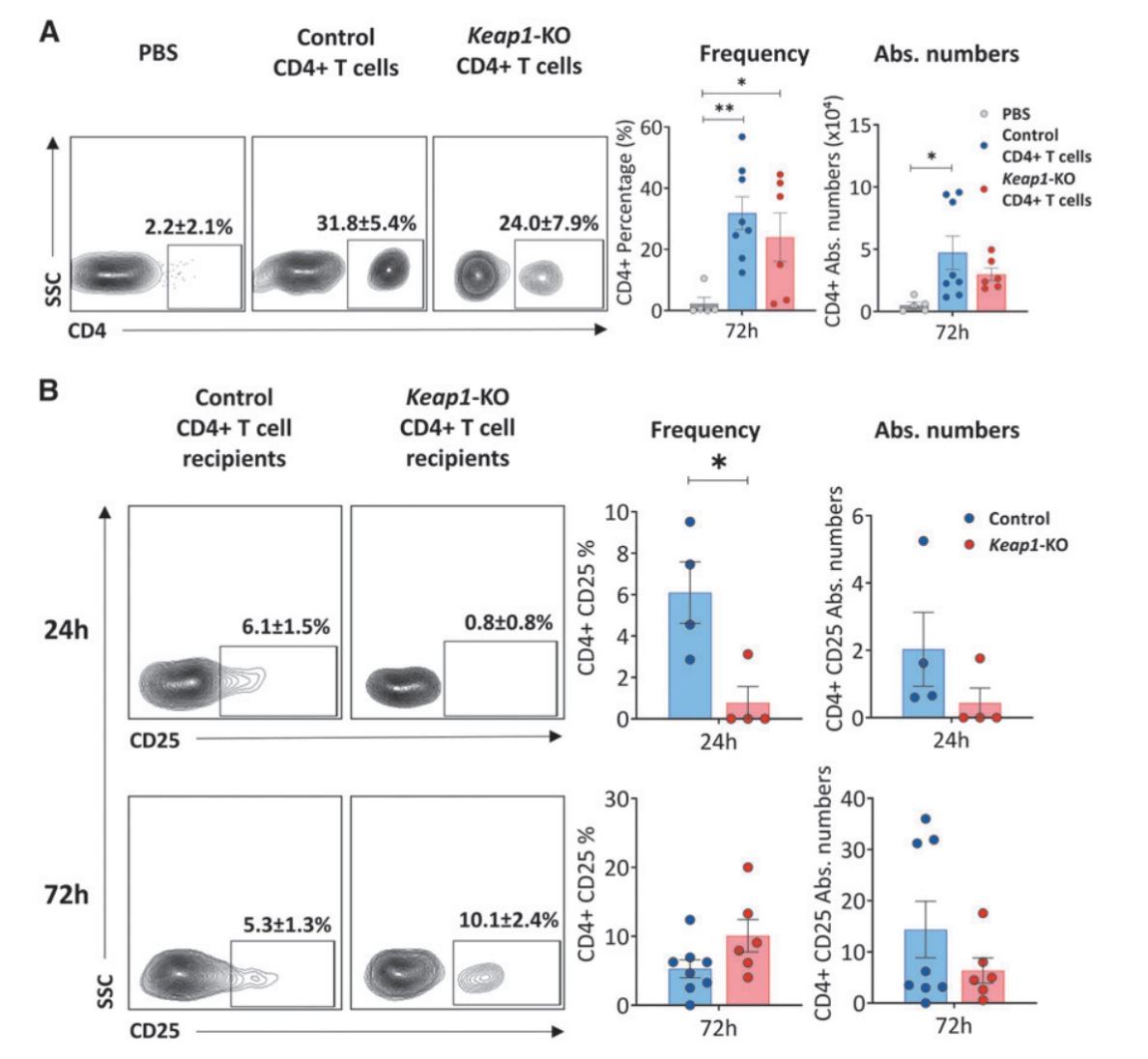
Fig.5 Confirmation of adoptive transfer and immunophenotyping of recipient kidneys
In conclusion, the study demonstrated the potential of CRISPR/Cas9-mediated gene editing to enhance Nrf2 activity in CD4+ T cells as a novel therapeutic approach for kidney IRI. The Nrf2/Keap1 pathway is a crucial therapeutic target for kidney diseases, by disrupting the Keap1 gene, which negatively regulates Nrf2, the researchers used CRISPR knockout technology to construct Keap1-KO CD4+ T cells with increased antioxidant capacity. Keap1-KO CD4+ T cells sustained Nrf2 activation and upregulation of antioxidant genes, while adoptive transfer of the cells into nu/nu mice with kidney IRI led to improved renal function.
Although further research is needed to address potential limitations, including off-target effects and the long-term consequences of T cell CRISPR gene editing, this study provides a promising foundation for the development of T cell CRISPR gene editing-based therapies for kidney diseases.
The findings highlight the potential of CRISPR/Cas9 technology to precisely knockout genes, and create genetically engineered cells with desired therapeutic properties, offering new insight for treating various kidney diseases.
EDITGENE has innovatively developed an efficient gene knock-out technology, offering customized gene knock-out services using an upgraded CRISPR/Cas9 system. With over a decade of experience in gene editing, EDITGENE has refined the optimal gRNA and homology arm design strategies, ensuring that their gene knock-out services deliver higher positive rates and broader applicability across various gene loci!
Recent Blogs:
Follow us on social media
Contact us
+ 833-226-3234 (USA Toll-free)
+1-224-345-1927 (USA)
info@editxor.com






