[Research Frontier] New trends in CRISPR-- Kras, NLRP3 and BRAF knockout cell lines
CRISPR/Cas9 knockout cells has a wide range of applications in life sciences, medicine and drug discovery, such as to generate disease models, drug screening and target validation, and knockout of disease-causing genes to achieve gene therapy for diseases. In this article, we have selected three studies on gene KO cells to explain to you the latest progress of this technology.
Kras gene knockout cells provide a new approach for the treatment of pancreatic cancer
https://doi.org/10.3390/ijms20225706
Pancreatic cancer is a kind of malignant tumor with high invasiveness and mortality. In 90% of cases, there is genetic variation of Kras proto oncogene, which leads to uncontrolled proliferation of cancer cells. Although the fight against pancreatic cancer continues, targeted therapy for Kras has always been challenging. In recent years, scientists have discovered a gene editing system called CRISPR/Cas9, providing new hope for cancer treatment.
Researchers used the CRISPR/Cas9 system to knockout cells carrying c.35G>A (p.G12D) Kras mutations to investigate and understand the role of Kras mutations in the occurrence and development of pancreatic ductal adenocarcinoma. The knockout of KrasG12D was validated through DNA sequencing and Western blotting. The results showed that under normal growth conditions, Kras knockout cell line and wild type cells were similar in function and expression. Further research on the key signal transduction pathway of Kras activation found that PI3K/Akt overexpression in human and mouse Kras gene knockout cells showed different expression patterns, revealing the complexity and diversity of signal transduction and survival mechanisms of pancreatic cancer cells. Kras knockout cells can help researchers understand the role of Kras in pancreatic cancer, develop new treatments, and more effectively fight against pancreatic cancer.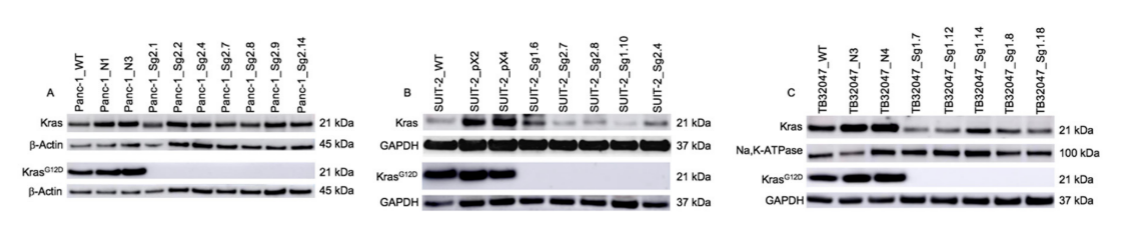
Fig1. Different protein expression patterns of human and mouse Kras gene
Knockout of NLRP3 gene reveals the key role of viral glycoproteins in triggering immune responses
https://doi.org/10.3390%2Fv13102076Virus infection and its related complications have become a major global health issue. Viral infections can cause various diseases with varying symptoms, ranging from asymptomatic to chronic, acute to fatal, reflecting the diversity and biological characteristics of viruses. Deeply studying the viral factors that trigger immune responses is crucial for understanding the pathogenesis of viruses. As a part of the innate immune system, inflammasomes can be activated by viral pathogens. However, the viral structural components responsible for activating inflammasomes are still largely unknown.
This study analyzed viruses such as SARS-CoV-1/2, HCMV, and HCV. The researchers used CRISPR-Cas9 technology to knock out the NLRP3 and GSDMD genes in THP-1 macrophages and understand their roles in viral glycoprotein induced inflammatory response. The experimental results showed that viral glycoproteins can strongly induce the activation of NLRP3 inflammasomes and cell pyroptosis, while knockout of NLRP3 and GSDMD significantly inhibits these responses. These results not only reveal the mechanism of viral glycoproteins as innate immune triggers, but also provide new perspectives for vaccine development and vaccine induced innate immune research. NLRP3 Knockout cells provide new insights and impetus for understanding the pathogenesis of viruses and developing new vaccines.

Fig 2. Knockout of NLRP3 gene inhibits activation of inflammasomes and cell pyroptosis
CRISPR-Cpf1 mediated BRAF knockout reveals potential for precise cancer treatment
https://doi.org/10.1016/j.omtn.2017.05.009
BRAF-V600E is one of the most reported driver mutations among various cancers, with this mutation present in all patients with hairy cell leukemia, 95% of patients with papillary craniopharyngioma, approximately 45% of patients with papillary thyroid cancer, and over 50% of patients with melanoma. However, the efficacy of BRAF inhibitors is limited to a subset of patients, and drug resistance and cancer recurrence remain major issues in clinical practice.
Researchers used CRISPR-Cpf1 (i.e. CRISPR/Cas12a), CRISPR-Cas9, and CRISPR-Cas9 EQR system technology to knockout the V600E mutated BRAF gene and investigate its role in cancer occurrence and development. They then compared the performance and application of the two gene editing systems in cells. The results showed that Cpf1 mediated cell death after gene editing, as well as a significant decrease in BRAF and pERK1/2 protein expression, Cas9 was able to recognize and cleave normal and mutant alleles, while the EQR variant did not observe significant gene editing events, indicating the potential applicability of cpf1 in precision medicine. BRAF knockout cell line provides valuable information for studying BRAF inhibitor resistance, cancer recurrence, and developing new therapeutic strategies. By conducting gene editing experiments in this cell line, researchers can better understand the application prospects of Cpf1 in precision medicine, especially in gene therapy for specific cancer-related mutations.
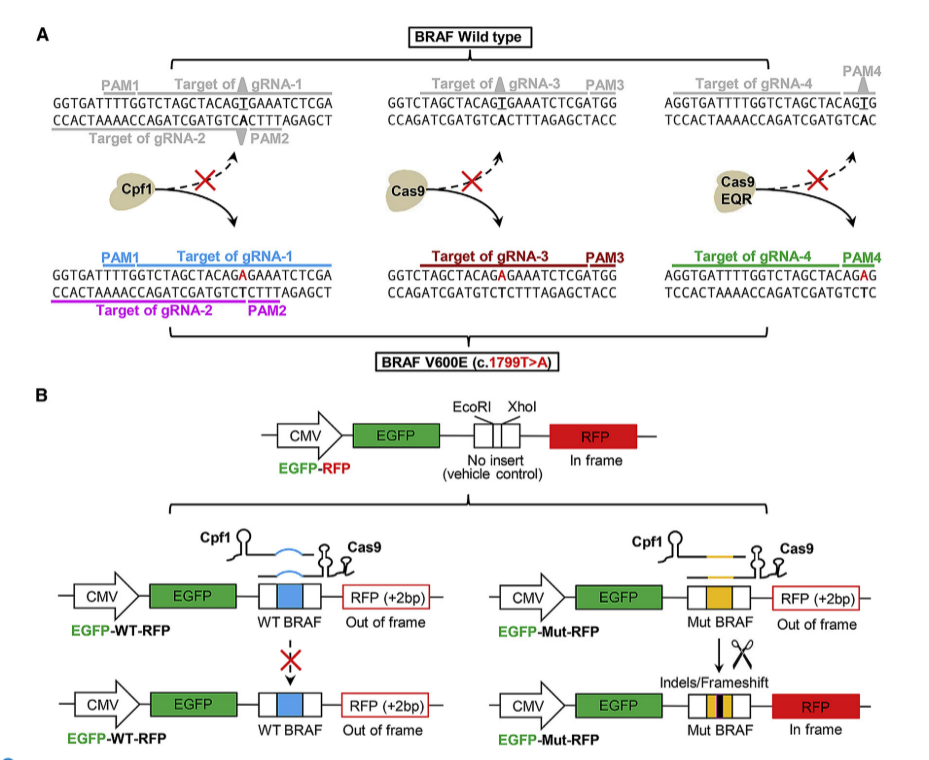
Fig. 3 Comparison of Knockout Effects of BRAF-V600E in Cpf1, Cas9, and Cas9 EQR Systems
EDITGENE offers 3800+ pre-made KO cell lines , including the aforementioned Kras, NLRP3 and BRAF knockout cells. Only $1900!
Recent Posts
[Advanced Materials] Cas13 provides a new strategy for immunotherapy of tendon injuries
[Nature] CRISPR Point Mutation Cells Help Deep Whole-genome Analysis
[FEB Bestselling] CRISPR Screen for Metabolic, Transcription Factor, RNA-Binding Protein
Contact Us

