[Research Frontier] New trends in CRISPR-- KO Cells Reveal Key Factors in Ferroptosis
CRISPR/Cas9 knockout cells has a wide range of applications in life sciences, medicine and drug discovery, such as to generate disease models, drug screening and target validation, and knockout of disease-causing genes to achieve gene therapy for diseases. In this article, we have selected three studies on gene KO cells to explain to you the latest progress of this technology.
1. FKRP KO Therapy shed a light on LGMDR9 Disease Treatment
Limb-Girdle Muscular Dystrophy R9 (LGMDR9) is a rare neuromuscular disease caused by defects in the Fukutin-related protein (FKRP) gene leading to a lack of glycosylation of α-DG, which in turn disrupts the integrity of the cell membrane. Currently, gene therapy using recombinant adeno-associated virus (rAAV) as a vector has become an important means of treating such diseases, providing an effective therapeutic strategy for this type of neuromuscular disease.
In the clinical trial phase, to demonstrate the safety and efficacy of the therapeutic product, researchers used CRISPR-Cas9 technology to create FKRP knockout cells, which were completely lacking in α-DG glycosylation, and developed a high-throughput On-Cell-Western technique to assess the effect of the gene therapy on the restoration of α-DG glycosylation in the knockout cells, thereby quantify the biological activity of the FKRP transgene, by which the half-maximal effective concentration (EC50) can be determined to compare different batches of rAAV-FKRP products. Mimicking the LGMDR9 disease state by FKRP knockout cells provides a powerful tool for studying LGMDR9 disease by better understanding the pathogenesis and potential treatments for the disease.
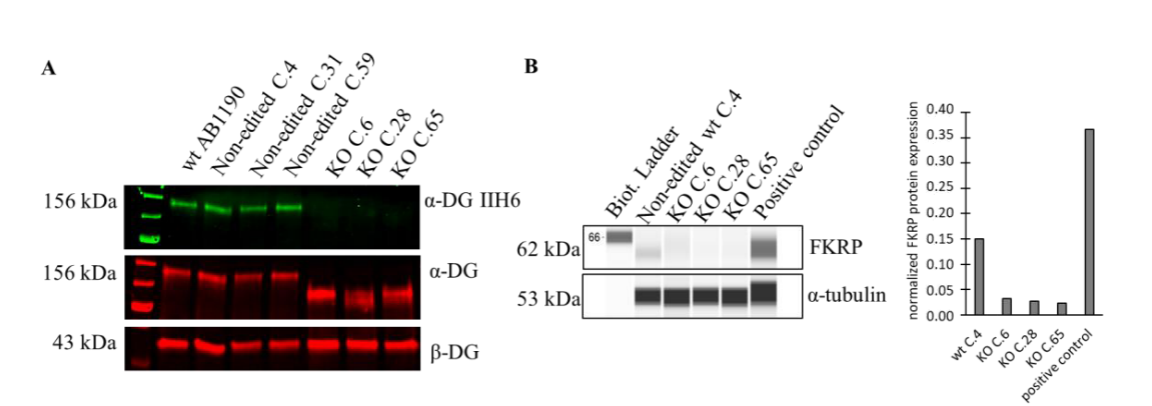
Fig 1. Reduced alpha-DG protein levels in FKRP knockout cells
2.Discover the Mystery of Sepsis: METTL3 KO Cells Reveal Role of NETs and Ferroptosis
Neutrophil extracellular traps (NETs) play an important role in sepsis-induced acute lung injury (SI-ALI). NETs are released by polymorphonuclear neutrophils, which have potent antimicrobial properties but are also involved in exacerbation of the condition in infectious diseases such as sepsis. In this paper, we found that NETs trigger SI-ALI in alveolar epithelial cells by inducing ferroptosis. In patients and model mice with SI-ALI, the excessive release of NETs is accompanied by enhanced ferroptosis, a process that is dependent on METTL3-mediated m6A modification of HIF-1α and subsequent reprogramming of mitochondrial metabolism.
Researchers compared METTL3 overexpression and knockout cells by RNA sequencing and MeRIP-seq techniques, and found that NETs-induced SI-ALI symptoms were alleviated and NETs-induced ferroptosis was significantly suppressed by METTL3 KO cells, suggesting that METTL3 plays a key role in NETs-induced lung injury. METTL3 affects NETs by regulating ferroptosis. This study demonstrated that METTL3 plays a key role in NETs-induced SI-ALI and ferroptosis, and the METTL3 knockout cells helped researchers to gain insight into the mechanism of NETs in SI-ALI and provided new ideas and methods for future treatment.
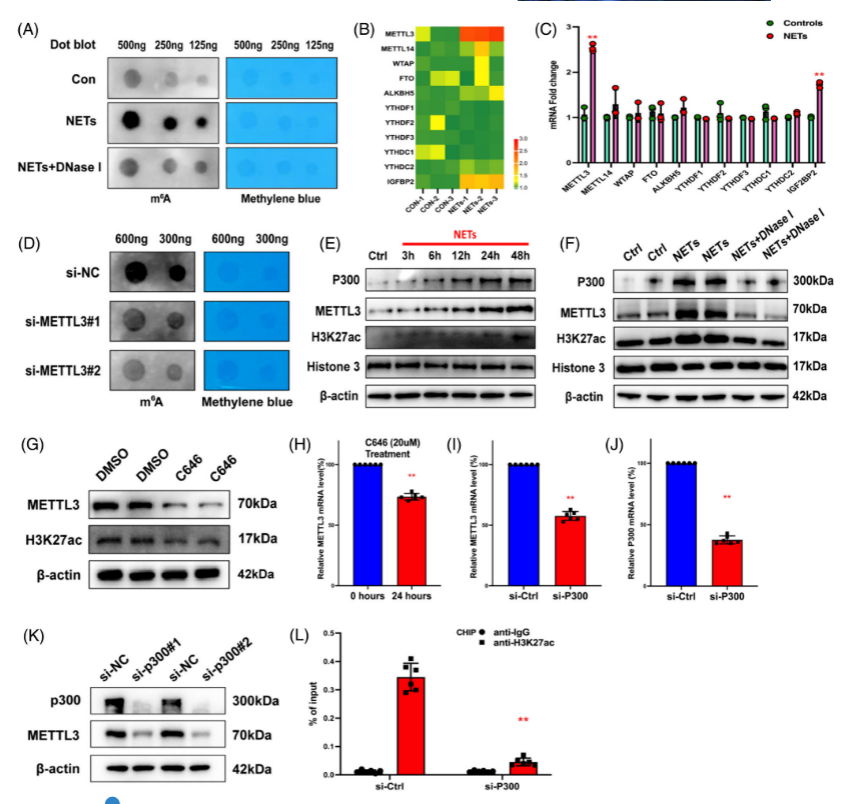
Fig. 2 METTL3 is important for SI-ALI and ferroptosis regulation
An effective immune response is essential for host defense; however, recruitment and dysregulation of immune cells can lead to inflammatory responses in many pathological conditions.PMNs are effector cells specialized to deal with the challenge of infection, but their accumulation and activity in tissues can lead to exacerbation of inflammation and tissue damage.A series of adhesion steps are required for PMNs to cross the endothelium, and there is a large population of resident macrophages in the intestinal mucosa. They are uniquely protective, releasing antimicrobial peptides and other chemicals to fight pathogen invasion; however, intestinal inflammation can disrupt this balance, leading to over-recruitment and activation of PMNs, further exacerbating the inflammatory response.
To understand how intestinal macrophages regulate PMN recruitment and function, researchers obtained vascular-associated macrophages (VAMs) from sites of infection or inflammation, and knockout the specific gene TNF-α in VAMs using the gene editing technology CRISPR-Cas9, and observed the processes of adhesion and signaling between PMNs and VAMs before and after gene knockout. After knockout of the TNF-α gene, VAMs released fewer chemokines, leading to a decrease in the recruitment efficiency of PMN.Compared with the control group, the inflammatory response was significantly reduced in the knockout group, and the tissue damage was reduced. TNF-α knockout cells in this study revealed the role of TNF-α in the regulation of PMN-EC interactions and the process of TEM, which has a significant effect on the vascular-associated macrophages' in-depth study and provide new therapeutic strategies for the treatment of diseases associated with excessive inflammatory response.
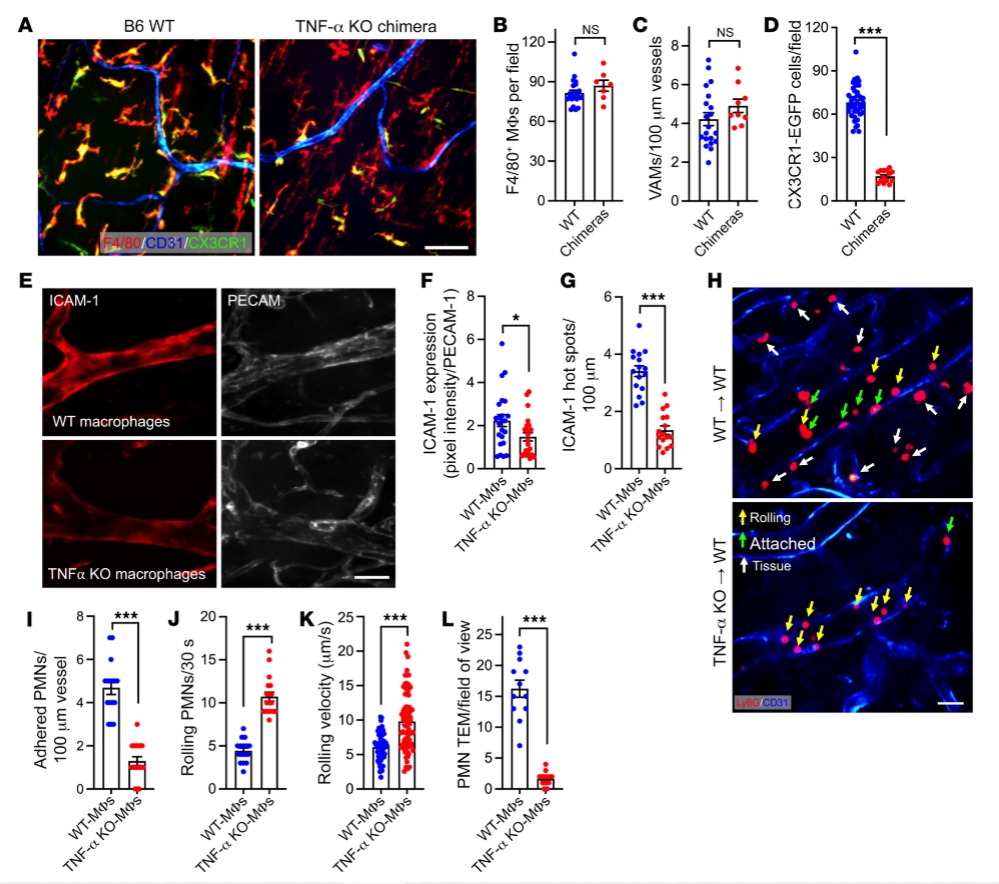
Fig.3 TNF-α knockout cells and control WT macrophage chimeras to detect TNF-α and PMN TEM association
EDITGENE offers 3800+ pre-made KO cell lines , including the aforementioned FKRP, METTL3 and TNF knockout cells. Only $1900!
CRISPR screen decodes stimulatory responses of primary T cells
New trends in CRISPR detection: Monkeypox virus detection
[DEC Bestselling Products] GLP1R, CTNNB1, PKM Knockout Cell Lines
Contact Us
Email:info@editxor.com
Phone:833-2263234 (USA)

