【Monthly Star】AMPK,TREM2 and p53 KO cell lines
Want to have a unique proposal for your research? Want to update the academic trends to keep your ideas sharp? Want to obtain valuable and cost-effective cell lines for deeper research? We collect our popular and inspiring Monthly Star: the 3 pre-made cell lines for your preference.
The protein encoded by this gene belongs to the ser/thr protein kinase family. It is the catalytic subunit of 5'-prime-AMP-activated protein kinase (AMPK).
AMPK is a eukaryotic energy sensor and its kinase activity is stimulated by an increase in the cellular AMP/ATP ratio. Phosphorylated AMPK regulates the activity of many key metabolic enzymes and it protects cells from stress from ATP depletion by cutting down ATP-consuming biosynthetic pathways. Alternative spliced transcript variants encoding different isoforms have been observed.
This September, University of Paris-Saclay in France published an article in the journal PloS one about the cardiac response to adrenergic stimulation in AMPKα2 knockout mice or the effects of pharmacological AMPK activation, which limits the adrenergic pathway rate. The adrenergic system is a potent stimulus for enhancing cardiac blood output and may become detrimental when energy metabolism is impaired.
In AMPKα2 knockout mice, the expression of AC5 increased while the pharmacological activation of AMPK by 5-aminoimidazole-4-carboxamide ribosomes (AICAR) resulted in decreased AC5 expression and a 40% reduction in cAMP content compared with unstimulated rat cardiomyocytes. Additonaly, in cardiac dysfunction induced by experimental pressure overload, it showed that AMPK activation downregulates AC5 expression and attenuates adrenergic activity,indicating a compensatory energy conservation mechanism between AMPK and adrenergic pathways. Therefore, we investigated possible interactions between AMPK activation and β-adrenergic pathways in healthy and dysfunctional hearts.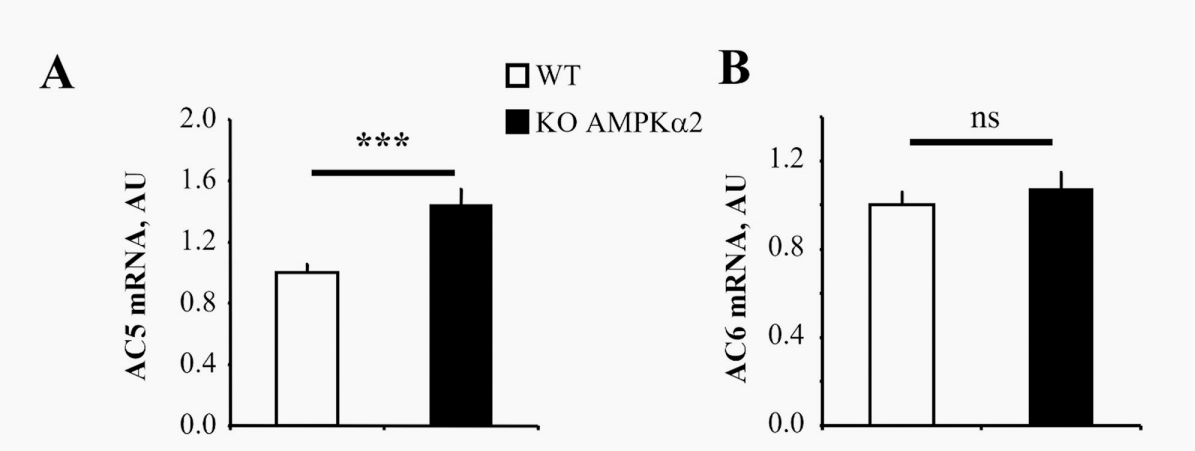
Figure 1: Expression of AC5 and AC6 in WT and AMPKα2-/- mice
Reference:
Modulation of cardiac cAMP signaling by AMPK and its adjustments in pressure overload-induced myocardial dysfunction in rat and mouse
The TREM2 gene encodes a membrane protein and with the TYRO protein tyrosine kinase-binding protein, the receptor signaling complex is formed. The encoded protein ppaticipates in immune responses and may be involved in chronic inflammation by triggering the production of constitutive inflammatory cytokines. Defects in this gene are responsible for polycystic lipomembranous bone dysplasia with sclerosing leukoencephalopathy. Alternative splicing results in multiple transcript variants encoding different isoforms.
This June, the First Affiliated Hospital of Sun Yat-sen University published a research paper. This research paper studied the important role of TREM2 knockout macrophages in inhibiting the infiltration of CD8 T cells after transarterial chemoembolization for hepatocellular carcinoma. Also, they explored the immune mechanism after transarterial chemoembolization (TACE) for hepatocellular carcinoma (HCC)
They collected 10 tumor samples(5 HCC patients who did not receive treatment and 5 patients who received TACE). The single-cell RNA sequencing was performed on them. An additional 22 paired samples were verified using immunofluorescence staining and flow cytometry. After TACE treatment, the number of CD8+ T cells decreased and the number of tumor-associated macrophages increased.
TREM2 is highly expressed in macrophages after TACE. Two models of TREM2 knockout mice and wild-type mice were co-cultured in vitro, which are the HCC cell orthotopic injection model and the spontaneous HCC model. Loss of TREM2 increased CD8+ T cell infiltration, thereby inhibiting tumor growth in two liver cancer models. More importantly, TREM2 deficiency enhanced the therapeutic effect of anti-PD-L1 blockade. These results indicated the reasons for recurrence of liver cancer after TACE and provide new targets for immunotherapy after TACE for liver cancer.
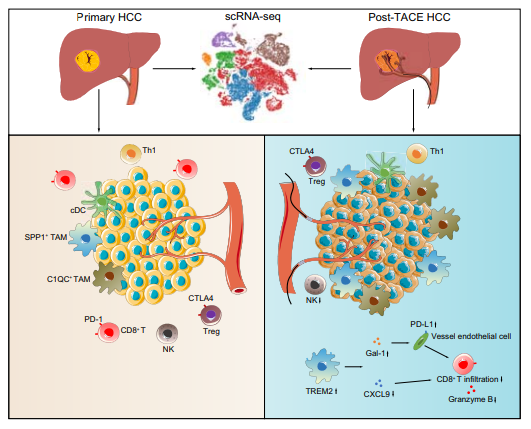
Figure 2: TREM2+ macrophages inhibit CD8+ T cell infiltration after transarterial
chemoembolization for hepatocellular carcinoma.
Reference:
TREM2 macrophages suppress CD8 T-cell infiltration after transarterial chemoembolisation in hepatocellular carcinoma ++
The P53 gene encodes a tumor suppressor protein that contains transcriptional activation, DNA binding, and oligomerization domains. The encoded proteins regulate the expression of target genes in response to different cellular stresses, thereby inducing cell cycle arrest, apoptosis, senescence, DNA repair, or metabolic changes. Mutations in this gene are associated with a variety of human cancers, and its alternative splicing and the use of alternative promoters of this gene result in multiple transcript variants and isoforms.
This July, University of Texas published an article in the journal Blood Advances, demonstrating that the loss of p53 enhanced the tumor-initiating potential and drug resistance of clonogenic multiple myeloma cells. Tumor recurrence and drug resistance are major factors limiting the curability of multiple myeloma (MM). Chromosomal deletion of p13 (del17p), which includes deletion of the TP53 gene, is a high-risk cytogenetic abnormality.
They found that loss of p53 increased the frequency of tumor-initiating MM cells (TICs) and drug resistance. Subsequent RNA sequencing (RNA-seq) studies showed that in p53 knockout (p53-KO) cells, the Notch signaling pathway was significantly activated and DNA binding inhibitor (ID1/ID2) gene expression was upregulated. They found that loss of ID1 or HES-1 expression or treatment with gamma secretase inhibitor (GSI) significantly reduced clonal growth of p53-KO but not p53 wild-type cells. In one set of MM samples, GSI treatment also reduced clonal growth in del17p samples but not in non-del17p samples. Because overexpression of Notch intracellular domain (NICD) restored the effect of GSI treatment. Their results support the potential of targeting the Notch1-Hes1 signaling pathway, including using GSIs to selectively reduce clonal growth and drug resistance in p53-KO cells.
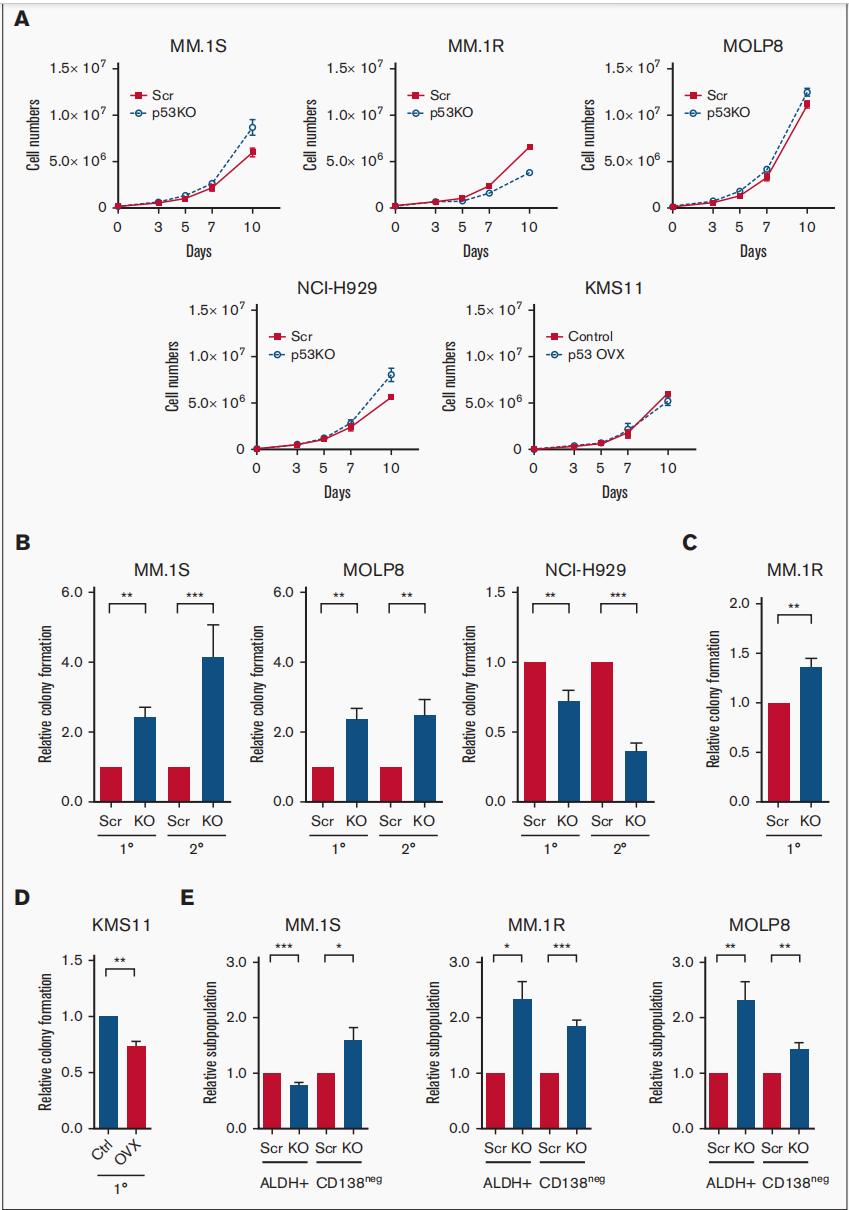
Figure 3: The tumor-initiating potential of MM cells enhanced by Loss of p53
Reference:
Loss of p53 enhances the tumor-initiating potential and drug resistance of clonogenic multiple myeloma cells
★We have the pre-made AMPK,TREM2 and Tp53 knockout cell lines,which are delivered in one week, just starting from $2000!Check the 3800+ Pre-made cell lines>>>
Email:info@editxor.com
Phone:833-2263234 (USA)
Recent Posts:
EDITGENE KO cells Decode SETD7 Regulation of TNBC Cell Migration and EMT Process Mechanism
[Research frontier] CRISPR Detection Trends

