【OCT Best Seller】XPO5, LRP6, RNF123 Knockout Cell Lines
Looking for an affordable cell model for your research?
Want to know the popular cell lines in nowadays research?
Here, based on the market trends of October, we listed the best sellers of this month. Hope this can provide inspiration for your research.
XPO5 Knockout Cells
XPO5 is a protein-coding gene, and it functions as a member of the nuclear protein family, which is necessary for transporting small RNA and double-stranded RNA binding proteins from the nucleus to the cytoplasm. The encoded protein relies on RanGTP as a transport carrier through the nuclear pore complex during the process.
The XPO5 gene is limiting the nuclear output rate of pre-miRNA and may be crucial for quantitative control of miRNA levels.
In an article published in the Journal of Nucleic Acid Research in September 2023, a novel role of lipid kinases in regulating miRNA levels in the study of Caenorhabditis elegans was validated. Identified the unrecognized PIP5K1A function in the biological origin of miRNA. Research has found that PIP5K1A regulates the ability of pre miRNA/XPO5 complexes to participate in miRNA biogenesis/output. In wild-type cells, PIP5K1A interferes with the interaction between pre miRNA and XPO5 to maintain normal levels of mature miRNA. And explained how PIP5K1A (reduced in PIP5K1A KD cells) allows more miRNAs to bind to XPO5 and output to the cytoplasm, leading to more mature miRNAs inhibiting downstream gene expression. Further investigation of its mechanism in human cells revealed that PIP5K1A interacts with the nuclear output protein XPO5 in the nucleus, regulating mature miRNA levels by blocking the binding of XPO5 to pre let-7 miRNA.
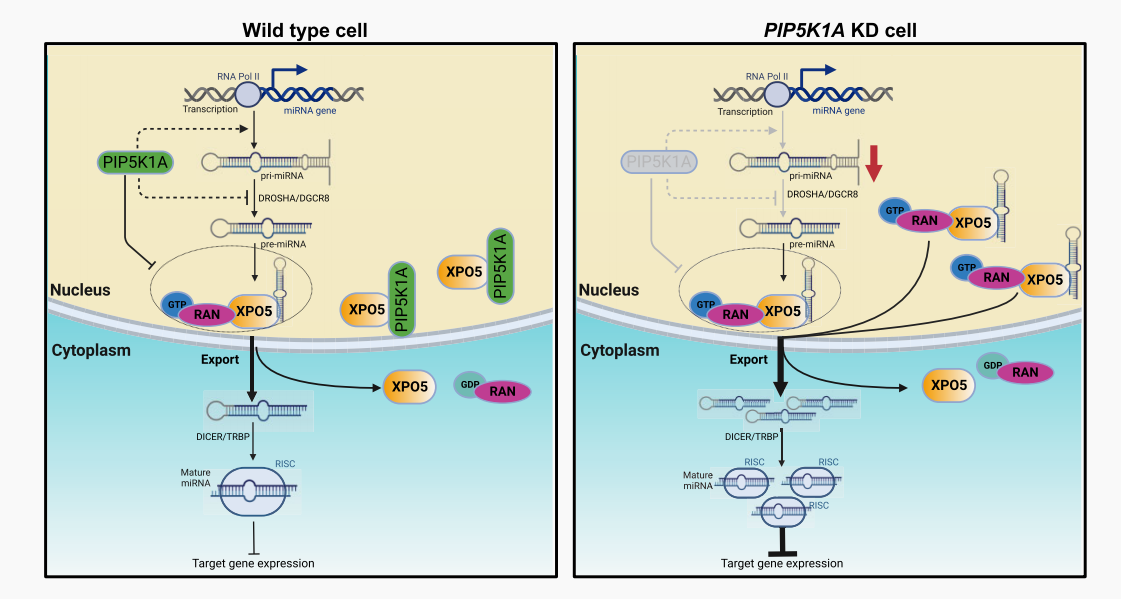
Model of PIP5K1A/XPO5 regulating pre-miRNA output from the nucleus to the cytoplasm
EDITGENE offers 3800+ pre-made knockout cells, XPO5 KO 293T cell line is available in-stock. Pre-made KO cells deliver in 1 week, only $1800. Explore our KO list now>>
LRP6 Knockout Cells
The LRP6 gene is a low density lipoprotein receptor related protein and one of the membrane protein receptors that mediate the Wnt/β-catenin pathway signaling. It plays an indispensable role in the interaction between odontogenic epithelium and mesenchymal tissue derived from cranial nerve crest.
An article published in Nature Communication found the role of LRP6 gene in the physiological control of Wnt signaling, and the relative abundance of Wnt receptors plays a crucial role in controlling Wnt signaling in tissue homeostasis and human diseases. Although the ubiquitin ligases of Wnt receptors exhibit good characteristics, the de-ubiquitination enzymes that reverse these reactions are still unclear. Here, it was found that USP46, UAF1, and WDR20 (USP46 complex) are positive regulators of Wnt signaling in human cells. In African Xenopus and zebrafish embryos, the USP46 complex is also required for Wnt signaling. The Wnt signaling pathway promotes the association between the USP46 complex and the cell surface Wnt co-receptor LRP6. The knockout of USP46 reduced the steady-state level of LRP6 and increased the level of ubiquitinated LRP6. On the contrary, overexpression of the USP46 complex blocked the ubiquitination of LRP6 by ubiquitin ligases RNF43 and ZNFR3. Based on this, a model is proposed where the USP46 complex increases the steady-state level of LRP6 on the cell surface and removes obstacles in the ubiquitin chain space through pruning mechanisms, promoting the assembly of LRP6 into signaling bodies.
This article reveals a potential mechanism by which the USP46 complex acts after LRP6 receptor activation and before signal body formation to control Wnt signals from the plasma membrane. Research has shown a new mechanism, namely the importance of regulating the steady-state level of LRP6 in the physiological control of Wnt signals.
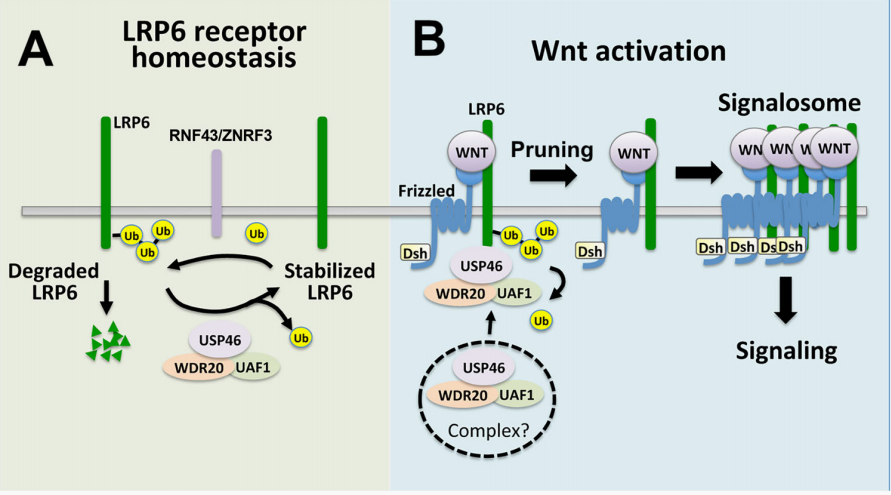
The regulation of LRP6 in the Wnt pathway by USP46 complex
EDITGENE provides pre-made KO cell lines and custom cell line service. Custom service starting from $2000. Get a quote>>
RNF123 Knockout Cell Line
The RNF123 gene is a protein-coding gene, which encodes a protein containing a C-terminal RING domain and an N-terminal SPRY domain. This domain exists in various proteins with different functions, and is known to participate in protein-protein interactions and protein-DNA interactions. This protein exhibits E3 ubiquitin ligase activity against cyclin dependent kinase inhibitor 1B (also known as p27 or KIP1). Variable splicing leads to multiple isoforms
In previous research, it was found that the cytokine signal transduction inhibitory factor (SOCS1) protein mainly undergoes ubiquitination of lysine (K48) connections. However, the E3 ubiquitin ligase that promotes the ubiquitination of SOCS1 (K48) connections is still unclear. In this study, it was found that ring finger protein 123 (RNF123) binds to the SH2 domain of SOCS1 through its RING domain, promoting ubiquitination of the k48 connections of K114 and K137 residues of SOCS1. Further research has found that RNF123 promotes the proteasome degradation of SOCS1, and through SOCS1, promotes the production of type I IFN mediated by totll-like receptor 3 (TLR3) - and interferon (IFN) regulatory factor 7 (IRF7) during duck smallpox virus (DTMUV) infection, ultimately inhibiting DTMUV replication. These findings demonstrate a new mechanism by which RNF123 regulates the type I IFN signaling pathway during DTMUV infection by targeting SOCS1 degradation.
In this study, it was first found that RNF123, as an E3 ubiquitin ligase, regulates TLR3 and IRF7 induced Type I IFN signaling pathway during DTMUV infection by ubiquitination of K114 and K137 residues targeting SOCS1 and proteasome degradation of SOCS1.
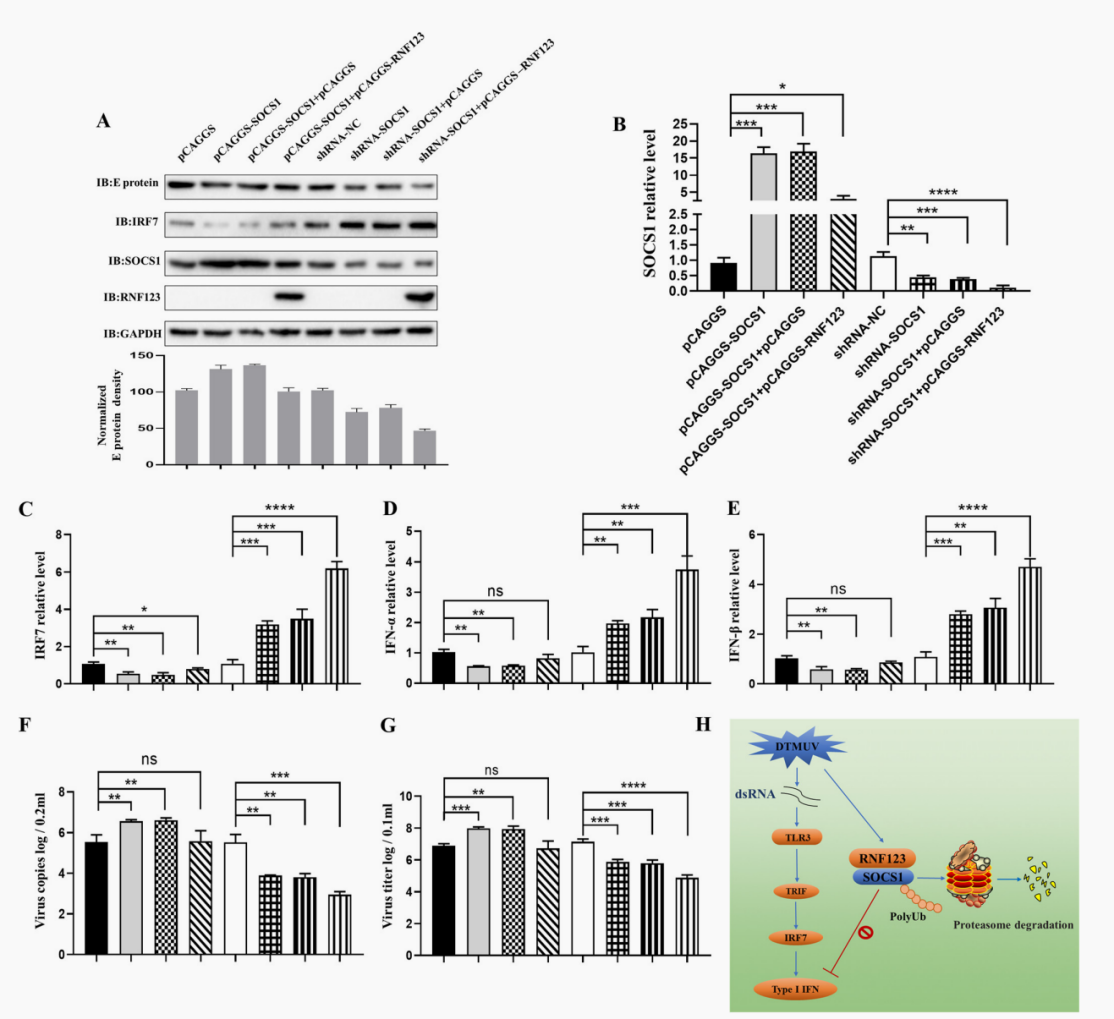
RNF123 affects the production of Type I IFN and inhibits the proliferation of DTMUV through SOCS1
Reference:
1.Lipid kinase PIP5K1A regulates let-7 microRNA biogenesis through interacting with nuclear export protein XPO5
2.The USP46 complex deubiquitylates LRP6 to promote Wnt/β-catenin signaling
3.RNF123 Mediates Ubiquitination and Degradation of SOCS1 To Regulate Type I Interferon Production during Duck Tembusu Virus Infection.
Recent Posts:
CRISPR Library Screening Applications
[Research highlight] Novel studies of CRISPR screening
How to utilize CRISPR library to speed up your research?

